

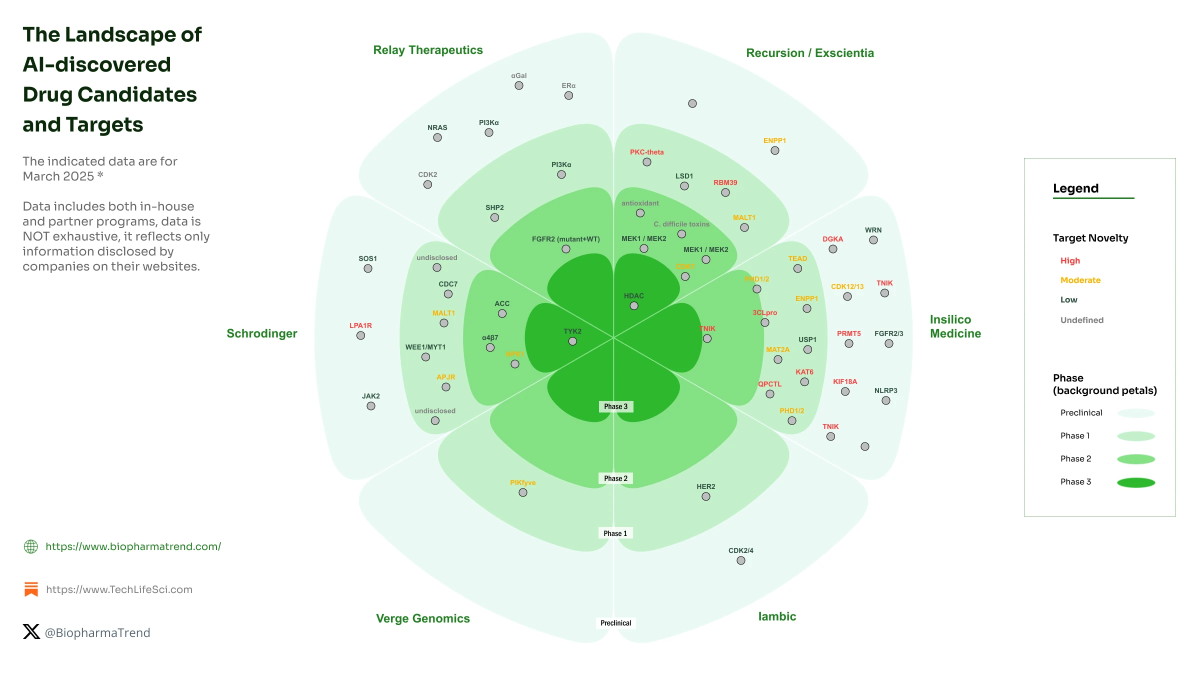
6
What it is: AI and machine-learning platforms are now designing biologic therapies (e.g., antibodies, proteins) which are entering human trials. For example, one AI-designed antibody (targeting TL1A for inflammatory bowel disease) entered Phase 1 dosing in 2025. Medium+1
Why it matters: This speeds up the traditionally long drug‐discovery cycle, reduces cost, and may enable targeting of previously undruggable proteins.
Key challenge: Translating AI‐generated candidates into safe and effective therapies in humans; managing regulatory, manufacturing and safety risks.
2. Next-Generation Gene Editing (Base/Prime Editing & In Vivo Delivery)

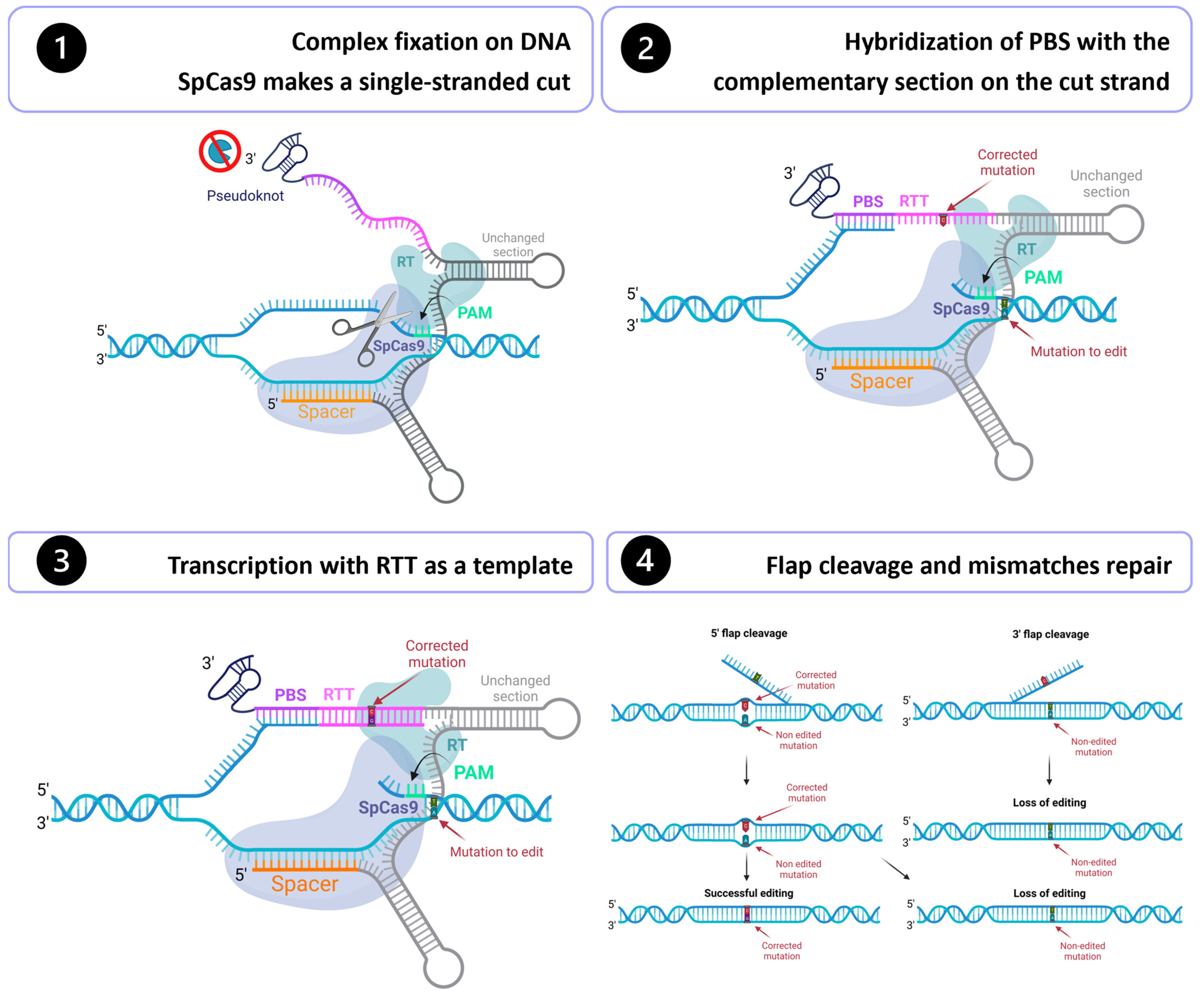
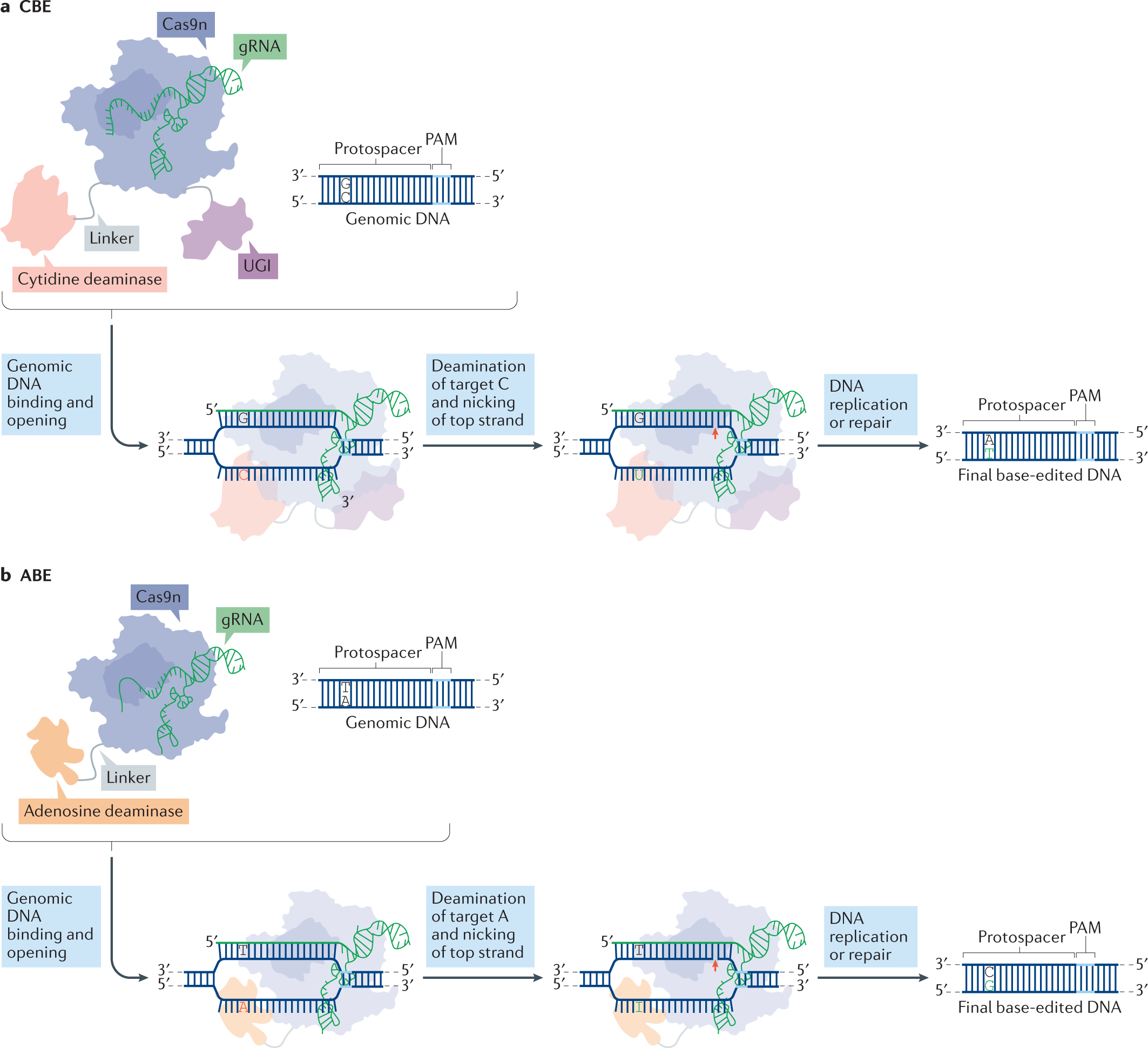
6
What it is: Beyond classic CRISPR-Cas9, newer gene-editing modalities (base editors, prime editors) allow more precise edits with fewer breaks. Also delivery technologies (lipid nanoparticles, engineered viral capsids) make in vivo editing more feasible. ewuet.com+2Medium+2
Why it matters: It means real possibility of curing single-gene disorders (and other conditions) with one treatment, rather than lifelong management.
Key challenge: Ensuring specificity, avoiding off-target effects, immune responses, long-term safety; also cost and access.
3. mRNA & RNA Therapeutics Beyond Vaccines

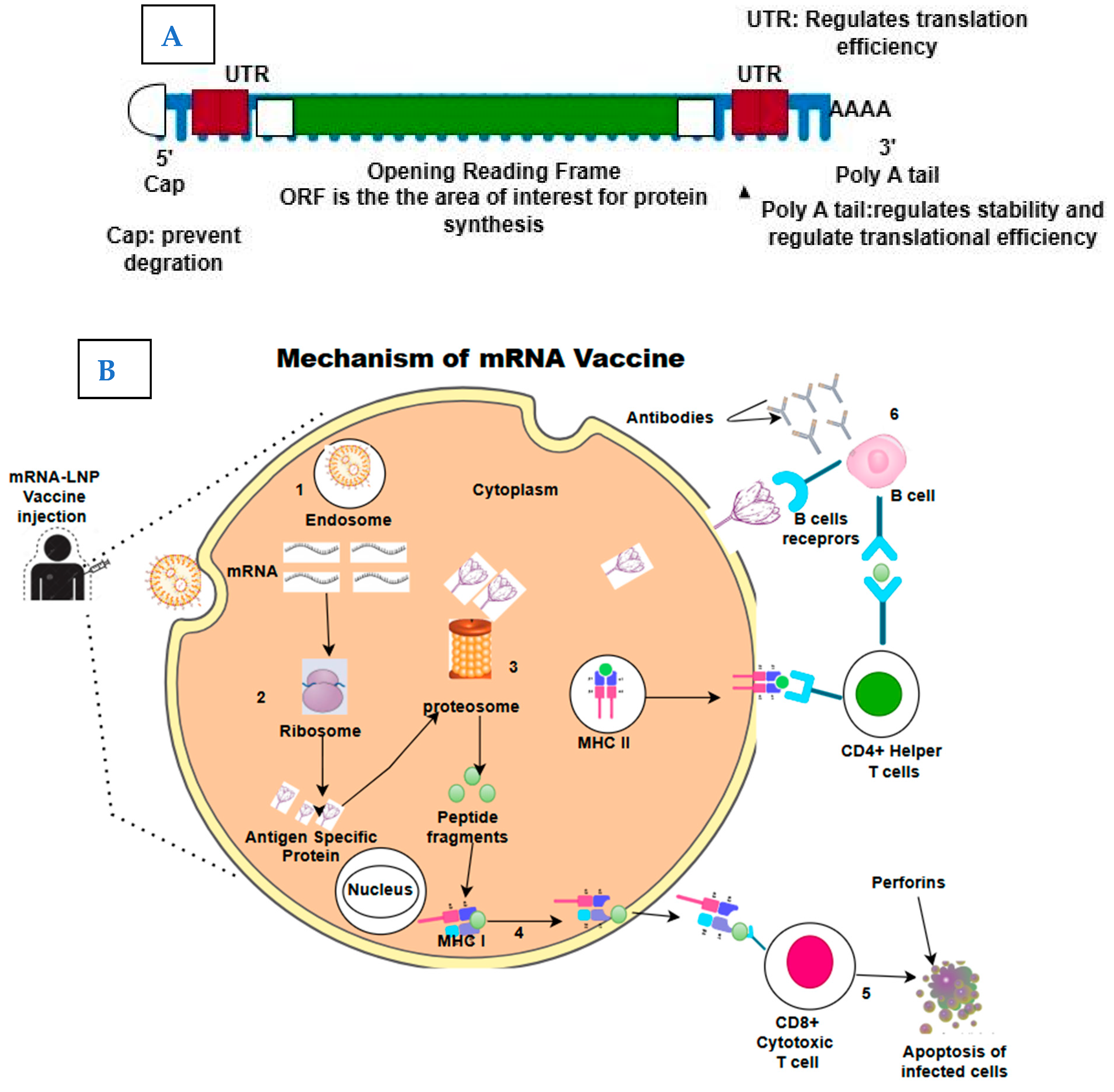
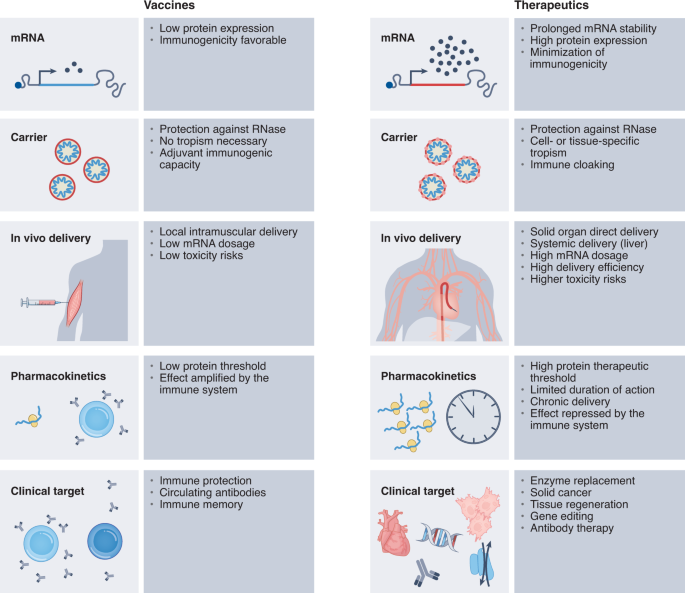
6
What it is: The mRNA platform, proven via COVID-19 vaccines, is now being expanded for therapeutic uses — protein replacement, cancer neoantigen vaccines, long‐acting secreted proteins. Medium+2Vygr News+2
Why it matters: Offers speed, modular design, and the ability to address diseases where traditional small molecules biologics struggle.
Key challenge: Delivery to the right tissue, durability of effect, cost, and immune reactions.
4. Advanced Cell Therapies & Off-the-Shelf “Allogeneic” Products
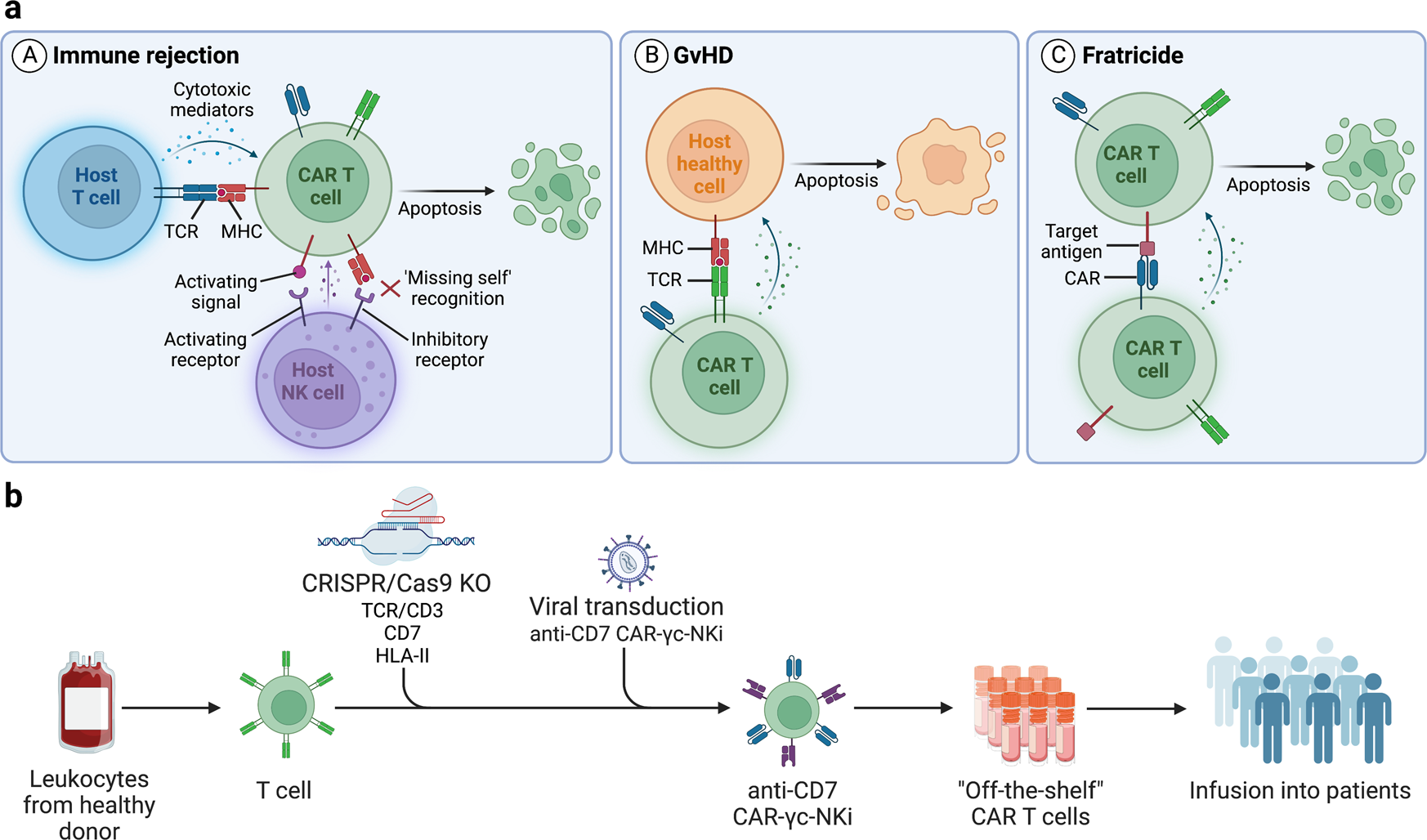
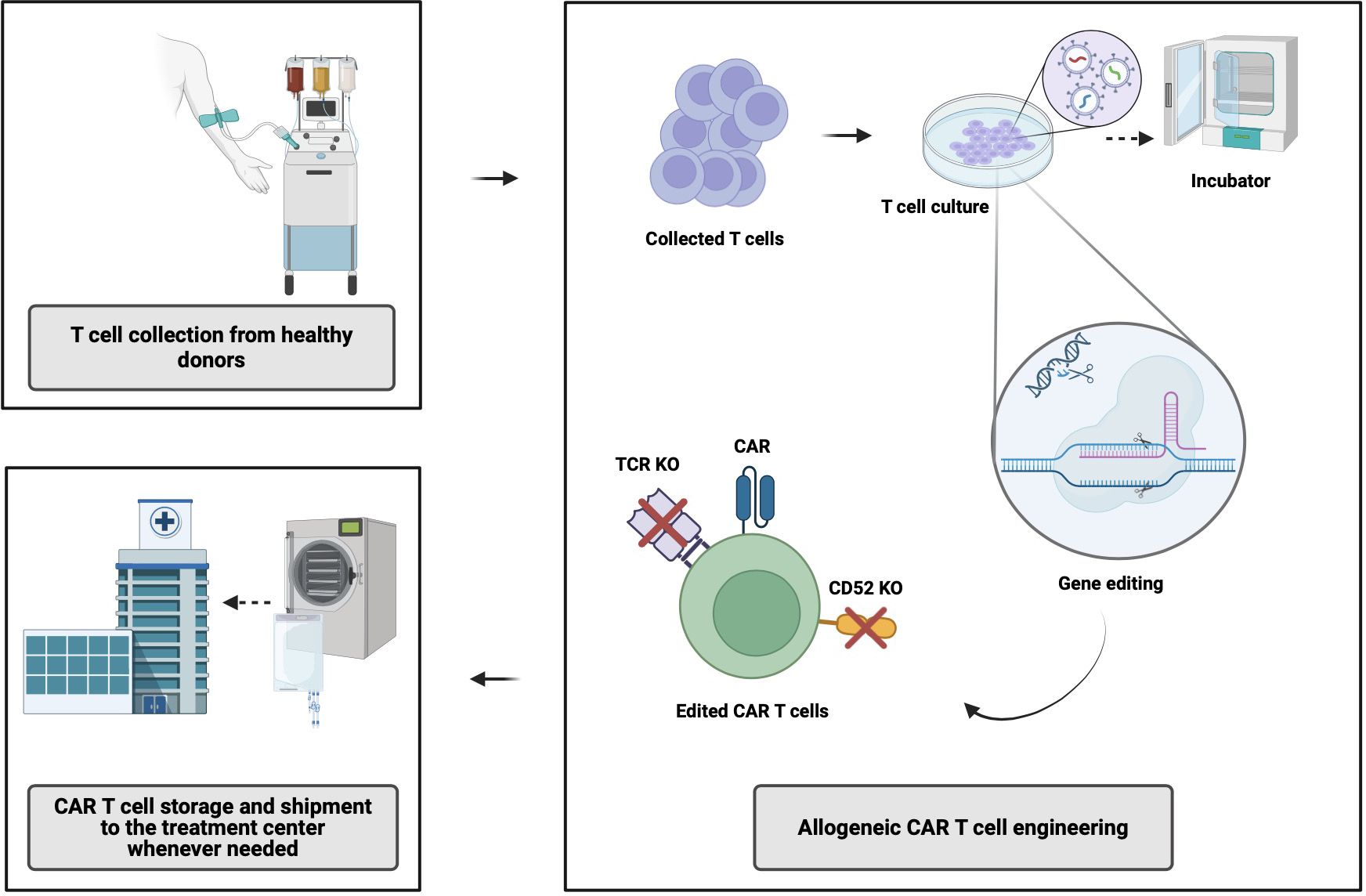
6
What it is: Cell therapies (like CAR-T) are moving from patient-specific (autologous) to donor-derived (allogeneic) models plus genome-editing to add safety switches. Medium+1
Why it matters: Greater scalability, reduced cost, broader access beyond rare cancers.
Key challenge: Immune rejection, manufacturing standardization, cost, regulatory hurdles.
5. Regenerative Medicine & 3D Bioprinting


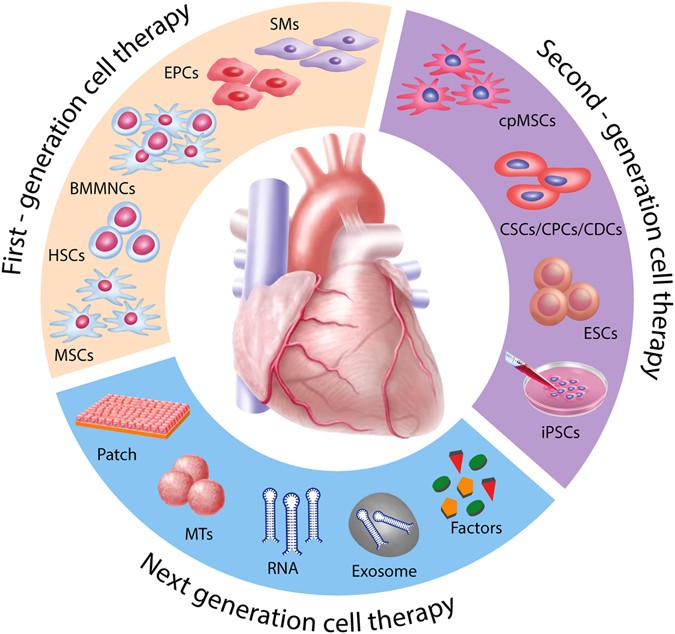
6
What it is: Using stem cells, scaffolds and 3D bioprinting to create tissues or organs for transplant, or repair damaged tissues (e.g., heart, liver, cartilage). Xtalks+2worldcaremagazine.com+2
Why it matters: Addresses major unmet needs (organ shortage, chronic degenerative disease) and shifts care from managing to truly repairing.
Key challenge: Vascularization of printed tissues, immune compatibility, scale manufacturing, regulation.
6. Liquid Biopsies & Multi-Omics Diagnostics

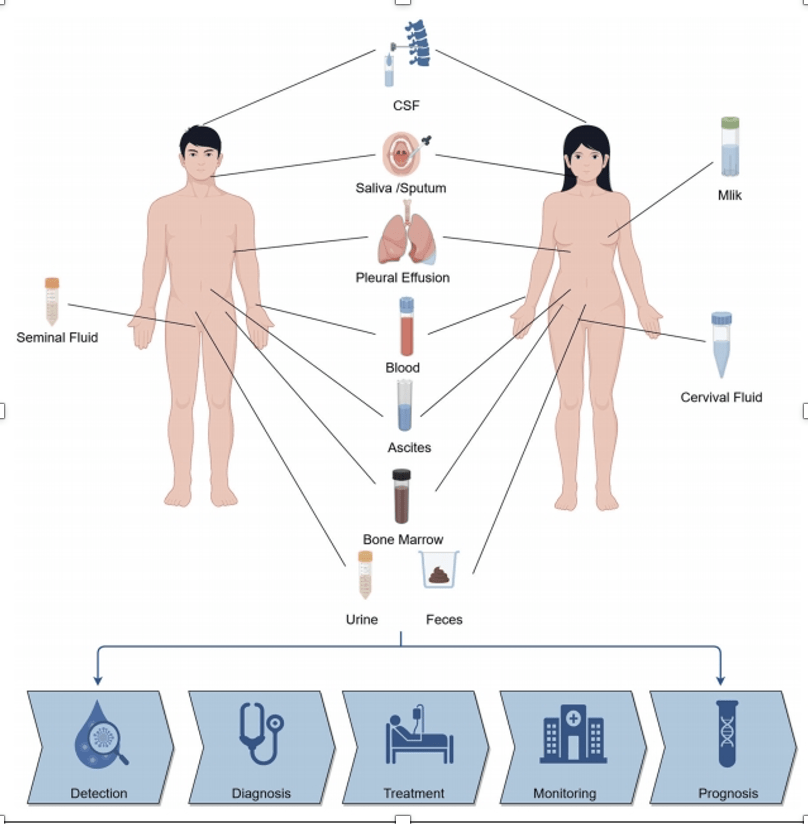

6
What it is: Non-invasive tests (blood or other fluids) using genomics, proteomics, spatial proteomics and AI to detect disease early (cancer, organ damage) or predict risk. aigen1.com+1
Why it matters: Early detection = better outcomes. Also enables monitoring of disease progression and personalized therapy.
Key challenge: Sensitivity/specificity in real-world settings, cost, data interpretation, and regulatory validation.
7. Wearables, Real-Time Monitoring & Digital Health Integration



6
What it is: Advanced wearable biosensors (heart rhythms, glucose, oxygen, other biomarkers) integrated with telemedicine and AI to continuously monitor health and intervene early. Vygr News+1
Why it matters: Moves healthcare from reactive to proactive; helps manage chronic disease, reduce hospitalizations, empower patients.
Key challenge: Data security, accuracy, user adoption, integration into healthcare workflows.
8. Synthetic Biology, Bio-Foundries & Automated “Cloud” Labs



6
What it is: Automated, scalable biotech workflows (“bio-foundries”, “cloud labs”) enable rapid design–build–test cycles for new biologics, cell therapies, diagnostics. Medium+1
Why it matters: Lowers cost, shortens timelines, democratizes biotech innovation.
Key challenge: Quality control, standardization, regulatory acceptance of automated-produced therapies.
9. Microbiome Therapies & Personalized Microbiome Modulation



6
What it is: Therapies targeting the human microbiome – e.g., restoring beneficial microbes, engineering microbes to deliver drugs, modulating gut flora to treat metabolic, immune or neurological disease. aigen1.com
Why it matters: The microbiome is increasingly linked to many diseases; offers new therapeutic avenues beyond genetics alone.
Key challenge: Complex biology, inter-individual variability, regulation (live microbes), safety.
10. Democratized Diagnostics & Therapeutics (Global Access, Low-Cost Platforms)


6
What it is: Innovations designed for cost-effective global health impact: thermostable vaccines that don’t require cold-chain, simplified diagnostics for low-resource settings, scalable manufacturing for global access. The Times+1
Why it matters: Bridging the health equity gap, enabling biotech benefits in low- and middle-income countries, addressing pandemics and global health threats.
Key challenge: Funding, distribution infrastructure, regulatory harmonization, ensuring local adoption and manufacturing.
Looking Ahead & Final Thoughts
These innovations are not merely “nice to have”—they reflect a paradigm shift in biotech and healthcare: moving from symptomatic treatment to prevention, repair and personalisation. They also reflect convergence: AI + biotech, digital health + therapy, automation + biology.
However, several cross-cutting issues remain:
- Regulation & safety: New modalities (gene editing, AI-designed biologics, microbiome therapies) challenge existing regulatory frameworks. arXiv
- Manufacturing & scale: Many therapies are still expensive, hard to produce at scale, and difficult to deliver globally.
- Ethics & access: Ensuring equitable access, avoiding biases in AI, protecting data privacy, and managing societal implications.
- Integration: Healthcare systems must integrate these new tools; clinicians, payers and patients must adapt.









Leave a Reply